美杏高德TPS系列--使用微孔板篩查生物素酶的缺陷
自動化TPS系列是中型開放式自動化移液工作站。一起自動化程度更高,自動更換吸頭,輕松助您完成高倍率梯度稀釋,單針挑樣、均一化,96/384/1536孔板等移液操作,并可整合振蕩器、孵育模塊等功能模塊。
以下是美杏高德使用TPS結合微孔板篩查生物素酶的缺陷實驗報告,請參考:
INTRODUCTION
Biotinidase enzyme is responsible for recycling endogenous biotin and plays an important role in processing protein bound dietary biotin. Without this enzyme Biotinidase deficiency develops and biotin cannot be recycled. Biotin supplementation is required for treatment in children diagnosed with Biotinidase deficiency. Clinical features of this disorder include seizures, skin rash, alopecia, hypotonia, ataxia, fungal infection, hearing loss, and vision problems. This disorder can result in developmental delay and metabolic compromise from ketolactic acidosis and organic acidemia and can be fatal1. Biotinidase deficiency is a treatable disorder with available screening tests and is therefore listed as one of the 29 primary targets by the American College of Medical Genetics. The incidence of this disease is greater than 1:75,000 births in the United States 2.
Nine states use Continuous Flow analysis (Astoria- Pacific International Clackamas, OR) for Biotinidase screening3. The purpose of this study is to evaluate the overall performance of the Biotinidase Microplate Reagent Kit (Astoria Pacific International, Clackamas, OR) using the SPOTCHECK® Express System. This system includes an automated pipettor (Apricot Designs, Covina, CA), incubator/shaker (Thermoscientific, Waltham, MA) and a microplate reader (Biotek, Winooski, VT) and is used for Biotinidase screening in newborns from dried blood spots and as an alternative to Continuous Flow.
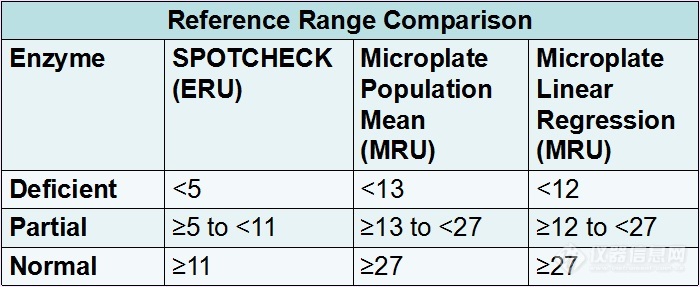
Figure # 1: Reference ranges comparison between the Continuous flow assay and the Microplate assay calculated from the normal population mean and the Microplate Assay calculated from Linear Regression
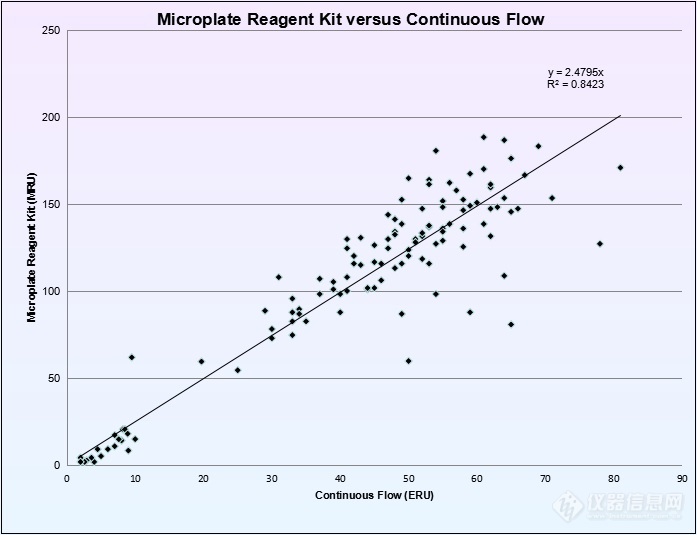
Figure #2: Linear regression analysis of Microplate Reagent Kit and the Continuous flow Assay
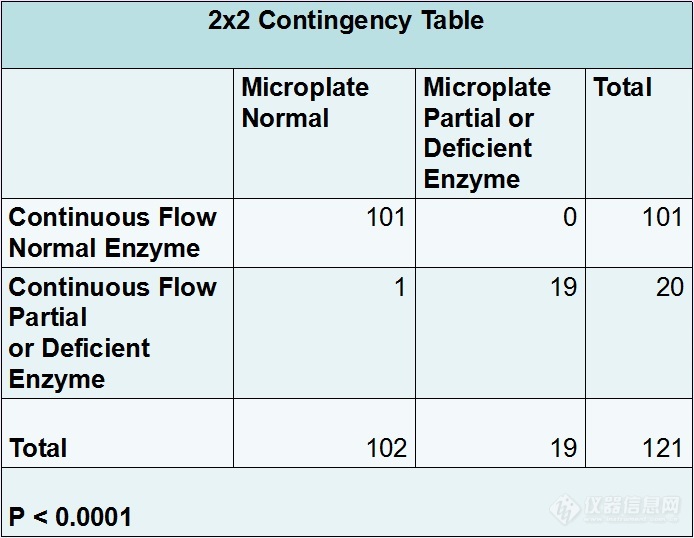
Figure #3: 2x2 Contingency table for Fisher’s Exact test
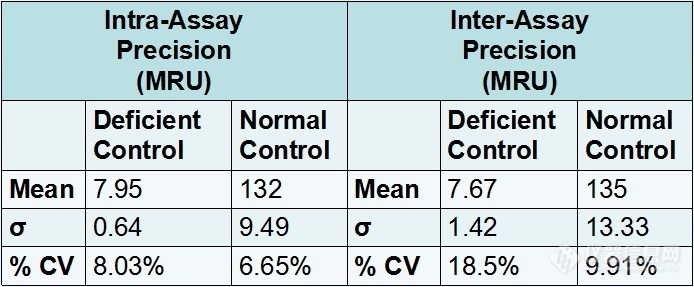
Figure #4: Precision study of the Microplate assay using the Apricot automated pipettor
The Microplate Reagent Kit identified one sample as normal that remained partial deficient by the Continuous flow assay. Through Fisher’s exact test and a 2 x 2 contingency table we calculated a two tailed P value of <0.0001. Therefore the outcomes of the two methods are extremely statistically significant.
Intra-assay and Inter-assay precision studies were conducted and were acceptable. There was %CV of 18.5% for the inter-assay precision using the deficient control, however the assay correctly identified the control as deficient every time.
Reportable range was evaluated using the kit standards ranging from 0 to 200 MRU. The product insert states “for sensitivity as little as 2 MRU biotinidase activity is discernable from no response. The limit of detection and limit of quantitation are both 3 MRU.” We identified deficient patients with values as low as 2.3 MRU using the Microplate assay.
Throughput for 288 specimens is five hours. Staggering runs by thirty minutes accommodates three runs per shift. The Microplate assay requires one filtration step and two transfer steps resulting in the use of four microtiter plates for every 96 specimens. The automated pipettor has stainless steel or disposable tips as an option. The assay is user friendly and maintenance is very minimal. Maintenance consists of a daily tip wash of the automated pipettor and refill of the DI water reservoir.
MATERIALS AND METHODS
Dried blood spot samples (n=121) exhibiting normal Biotinidase enzyme activity and confirmed cases of Partial and Complete Biotinidase deficiency were analyzed using the Biotinidase Microplate Reagent Kit to evaluate accuracy. Samples were analyzed following the manufacturer’s product insert and were run in tandem with our established procedure, Continuous flow analysis using the Biotinidase 50 hour reagent kit (Astoria Pacific International, Clackamas, OR).
Additional samples (n=2004) exhibiting normal enzyme activity were analyzed using the Microplate assay to establish reference ranges or cutoffs. Deficient and normal controls were used for intra-assay and inter-assay precision. For intra-assay precision we ran each control 20 times in one run. For inter-assay precision controls were run over a 20 day period.
Mean, standard deviation and %CV were calculated for each. Kit standard were used to verify reportable range. Data were assessed by linear regression, Fisher’s exact test and population statistics.
RESULTS
Newborn screening has identified a group of children with partial Biotinidase activity, between 10-30% of mean normal activity whereas below 10% of mean normal activity is profound Biotinidase deficiency.1,4 Our reference range using the Continuous flow assay is calculated at 10% of the normal population mean for Complete Biotinidase deficiency and 21% for Partial enzyme deficiency.
These same percentages were applied to derive reference ranges for the Microplate Reagent Kit.
The normal population mean for the Microplate Reagent Kit was 127 MRU (σ= 31.97 MRU, range = 25 to 288 MRU). Linear regression was used to calculate reference ranges for complete deficient, partial deficient and normal enzyme activity for comparison purposes. Due to a low volume of complete deficient and partial deficient enzyme samples compared to normal samples we forced the trendline to zero. Cutoffs from linear regression were similar to the percentage based reference ranges.
121 samples were evaluated for accuracy. 101 samples tested had normal enzyme activity by both methods (one sample was a confirmed partial deficient and the discrepancy is unexplained). 19 samples tested had abnormal enzyme. There was discord in this group of samples: four were deficient by the Microplate Kit but partially deficient by Continuous flow however abnormality was properly identified.
CONCLUSION
The Microplate Reagent Kit on the SPOTCHECK® Express System from Astoria-Pacific is a reliable method for Biotinidase screening in newborns. Tennessee adopted this methodology for use on August 1, 2009.
REFERENCES
Wolf B, Heard G, Pediatrics 85/4 512-517(April 1990).
Newborn Screening: Toward a Uniform Screening Panel and System, Genetics In Medicine 8/5 103S (May 2006).
Personal communication with Alana Lerch of Astoria Pacific International (June 2010).
4.Product Insert, Spotcheck Microplate Reagent Kit 60 plate, 4.9-WS-81-8000, (August 2008).